Flow Electrochemistry
Summary
Electrochemical synthesis is the use of electrical energy to drive chemical change; using electricity to replace toxic and costly chemical reagents. This allows cleaner and cheaper syntheses with greater production efficiency and at reduced cost.
Electrochemical Synthesis
In electroorganic synthesis, redox chemistry is carried out by interaction of the starting materials with the electrodes of the electrochemical cell. As electrons are essentially the reagent utilized for the redox process, the use of large amounts of often hazardous oxidizing or reducing reagents can be avoided. Reactive intermediates can be generated under relatively mild conditions (often room temperature) without producing any additional waste. Electrochemical reactions are therefore considered safe and provide some of the greenest and cost efficient synthetic strategies.
Organic electrochemistry deals with the synthesis of organic compounds by electrochemical redox reactions. Its origin can be seen in the Kolbe-electrolysis in 1849. Electrochemical synthesis methods have the outstanding advantage to allow reactions without "substantive" reagents, which otherwise would have to be separated in their used form from the reaction products. Because of the large oxidative and reductive potential range being directly accessible by electrochemical methods, they are in particular of great interest for industry for the production of organic compounds. Prominent examples are the production of adiponitrile, sebacic acid or esters thereof, hydroquinone, benzaldehyde, 4-aminophenol, piperidine, 2,5-dimethoxydihydrofuran, glyoxylic. acid and organic fluorine compounds.
Advantages of Electrochemistry
Access to chemical transformations not traditionally available through typical chemistries Reactivity can be “dialed-in” to achieve improved chemoselectivity.
Acceleration of redox steps in a complex catalytic cycle possible.
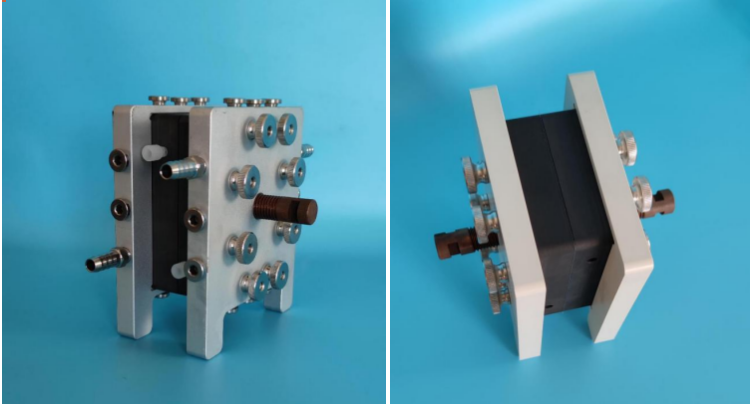
CM-ELFlow is our range of electrochemical cells and integrated systems. CM-ELFlow is a general purpose cell and is ideal for use in a R&D or laboratory settings for proof of concept work. The units are used by industrial, research and educational clients globally.MF-ELFlow is designed to be the most comprehensive, flexible and consistent electrochemical offering from lab scale to full production.
CM-ELFlow is an easy to use, flexible, hand-assembly laboratory electrochemical cell.The microflowidic electrochemical reactor has the characteristics of short electrode distance and continuous reaction. The feature of short electrode distance can bring advantages of low voltage, less amount of electrolyte or no electrolyte; continuous reaction can bring the advantage of no backflow of materials, so as to realize the product is not excessively redox and improve the product yield. In addition, the microchannel electrochemical reactor has the characteristics of accurate temperature control and fast mass transfer. Under the condition of extremely small electrode distance, special redox coupling reactions can also be realized.
Features
(1) Long residence time, which can realize continuous synthesis or processing;
(2) The temperature of the reactor can be controlled;
(3) There is a wide choice of electrode types, and the electrode distance can be adjusted;
(4) It can be carried out with or without diaphragm.
Continuous flow electrochemical reactor other features
1.The CM-ELFlow continuous flow electrochemical reactor is a more convenient and simple electrochemical reactor;
2.Special heating and refrigeration board design (water cooling joints, electrode heads);
3.It adopts slow-moving wire-cutting technology to form, which can control the electrode temperature -30-200 degrees Celsius;
4.Simple clamping, integrated design of electrode plate and chip, higher cost performance;
5.The current collector unit is omitted, the electrode spacing is 0.025-2mm on demand, and the chip pool depth is 0.05-2mm on demand. It can realize a single flow cell unit and a rectifier flow cell, and realize a single electrolytic cell reaction and a double electrolytic cell reaction;
6.Hand-tight assembly without tools, insulated screw connection, breakdown voltage up to 20kv;
7.The electrode material can be selected from isostatic graphite, 99.9 pure copper, foamed nickel, and the sealing strength can reach 20bar;
8.The membrane models used in the water/alcohol system, pure organic phase system, and dual-cell system are different. Please specify the system when ordering.
Process cases that can be realized by continuous flow electrochemical reactor:
Continuous flow electrochemical reaction process, cathode reduction reaction (one-way circulation), anodic oxidation reaction (one-way circulation), redox reaction (two-way circulation).
Application fields of continuous flow electrochemical reactor:
electrolytic cells, preparation of special pharmaceutical intermediates, preparation of energetic materials, etc.
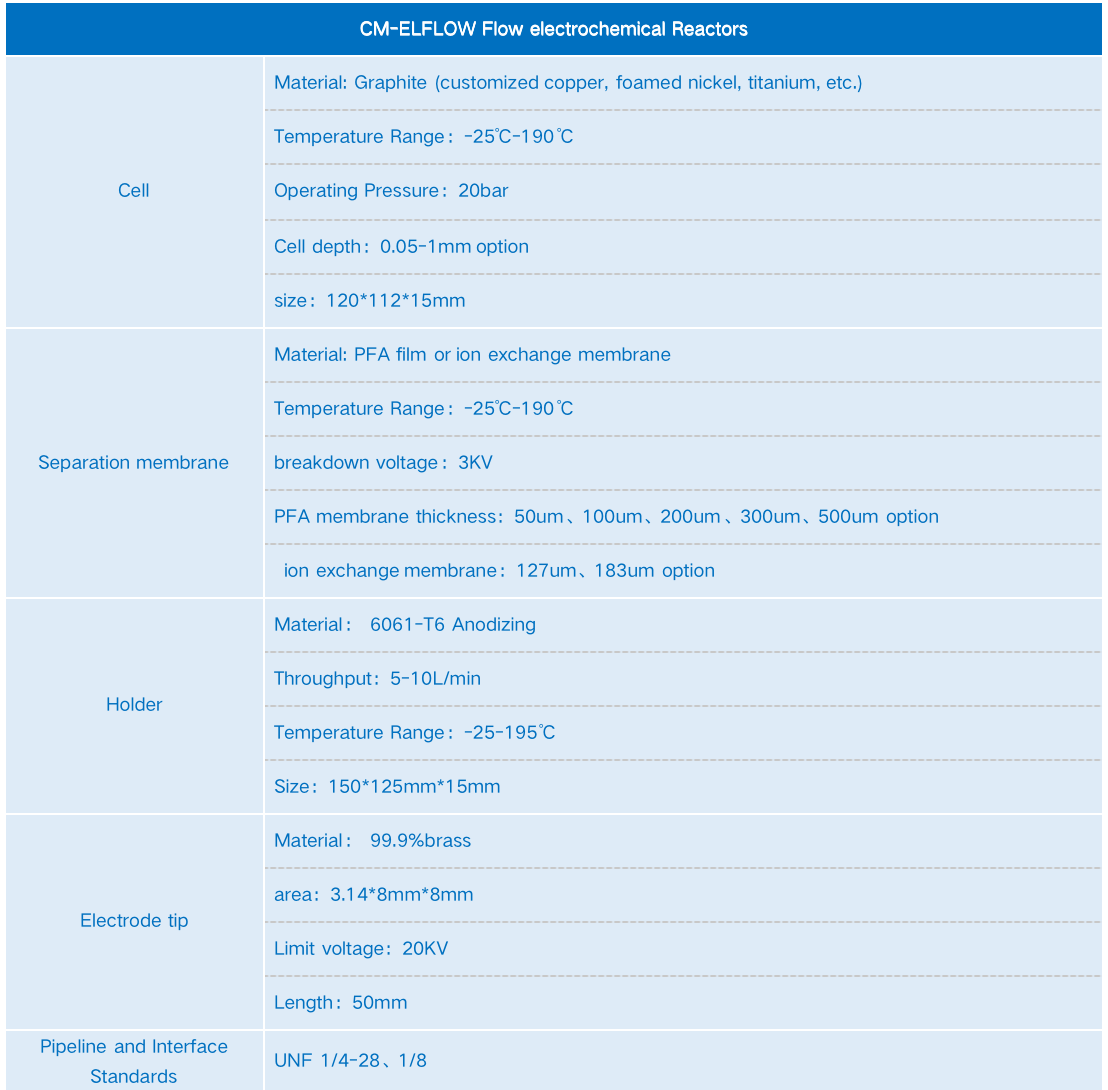
Technical Specifications for CM-Elflow Electrochemical Flow Reactor